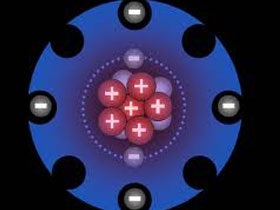
Dr. David Snoke explains How Electrons Orbiting the Nucleus Never Fall Into the Nucleus
- Ever since the Big Bang, the electrons inside every atom have been revolving their orbits for 15 billion years and they never collide with each other. Who maintains such an order within such a small realm?
- When we think about the electrons going around the atoms, this is actually one of the things that historically persuaded people that quantum mechanics was true. Because if you think of the electron as just this little billiard ball orbiting around the nucleus, sort of like the way the Earth orbits around the Sun, the problem with that is, as the electron moves it will be radiating energy away and eventually it will lose all of its energy and it will fall into the nucleus and crash. Just like we have a satellite around the Earth, none of them stay up there forever, they all eventually run out of energy and fall down and burn up in the atmosphere. There is a big question in people’s minds: why doesn't that happen with the electrons orbiting the atoms? And the answer turns out to be quantum mechanics:that the lowest resonance of the electron around the atom has a certain wavelength and it corresponds to a certain distance of electron away from the nucleus and it simply can't go any lower. So in terms of a musical analogy, you could say if I’m playing a musical instrument, there is one lowest note that I can play on that musical instrument, and I can't go any lower. We call that the fundamental resonance, something very much like that with the electrons around the atoms. You could say that the system of quantum mechanics is keeping the electrons stable, it is really another place where God intervenes to make the laws of physics work so that things allow us to exist and so on. If it was a purely classical world then actually we couldn't exist, because the electrons would just fall into the nuclei. And again when we talk about fine-tuning parameters, one of the parameters that isextraordinarily important is the Planck constant, which determines the length scale over which that quantum mechanics applies. If that was too large, then we would have really weird quantum mechanical effects and if it was too small then other bad things would happen. And so quantum mechanics is actually another example of a fine-tuned theory that allows things to be stable.